The past and present of a battery: the development and future of batteries
The battery is a great invention with a long and wonderful history and an equally brilliant future.

The battery has come a long way since its invention in 250 BC.
A battery, in essence, is a device that can convert stored chemical energy into electrical energy. Basically, a battery is a small chemical reactor that generates high-energy electrons through reactions to be injected into external devices.
The battery appeared earlier than we thought. In 1938, the director of the Baghdad Museum found the original battery now known as the “Baghdad Battery” in the basement of the museum. Analysis showed that this original battery can be traced back to 250 BC and belongs to the Mesopotamian civilization.
This earliest battery has sparked much debate. There are many theories about its purpose, including electroplating, pain relief, or that people used it to induce religious experiences through the tingling sensation of contact.
The American inventor Benjamin Franklin first used the word “Battery” in 1749, when he was experimenting with electricity using a group of capacitors connected in series.
The American inventor Benjamin Franklin first used the word “Battery” in 1749, when he was experimenting with electricity using a group of capacitors connected in series.
The wires connect the two ends of the battery stack to generate a stable current. Each small unit can generate an open circuit voltage of 0.76 volts (V). By connecting these small units in series, we can get a voltage equal to the sum of the voltages of each small unit.
The lead-acid battery is one of the longest-lasting batteries known, invented in 1859 and still used to ignite most internal combustion engine vehicles. It is also the earliest rechargeable battery.
Today, batteries can be large or small, from megawatt-level, used to store electricity from solar power plants or substations to ensure a stable energy supply in a certain area; to the size of a button, powering the electronic watch you wear.
Different batteries are based on different chemical reactions, which also makes each different small unit have a different open circuit voltage, usually between 1.0 and 3.6V. By connecting these small units in series, we can increase the voltage; and by connecting these small units in parallel, we can increase the current. This law is used by us to increase the voltage and current to provide the current and voltage we need. Even for megawatt-level batteries, its voltage and current are obtained through this most basic law.
Now it is predicted that battery technology will take another leap forward, with new battery models capable of harvesting enough energy from home solar and wind installations and storing it in sufficient capacity to power an entire home for days at a convenient time, usually at night.。
How Batteries Work
When a chemical reaction begins, extra electrons are released and the battery begins to discharge. An example of extra electrons being released is during the oxidation of iron to form rust, where iron reacts with oxygen, releasing electrons to oxygen to form iron oxide.
The standard battery construction is to separate two metals or compounds with different chemical potentials by a porous insulator. Chemical potential is the energy stored between atoms and chemical bonds. When electrons are free to move in the connected external device, this energy can be transferred to those moving electrons.
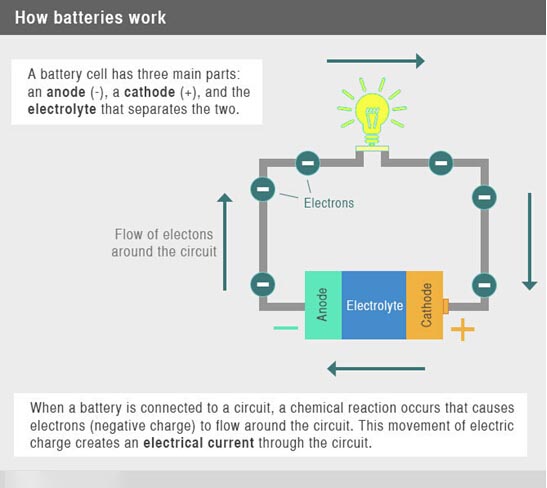
Conductive liquids such as salt water are often used to transport soluble ions. During the reaction process, these ions can be transferred from the surface of one metal to the surface of another metal in the solution. We usually call such conductive liquids electrolytes.
During the discharge process, the metal or compound that loses electrons is called the anode, and the metal or compound that gains electrons is called the cathode. In the external circuit, the flow of electrons from the anode to the cathode is the “current” we use to drive electrical devices.
Primary battery vs rechargeable battery
After generating current, some batteries cannot be reversed. We call these batteries primary batteries. When one of the reactants is consumed, the battery is no longer usable.
The most common primary battery is the carbon-zinc battery. This type of battery lasts longer if the electrolyte is alkaline. This is the alkaline battery we usually buy in supermarkets.

The difficulty with primary batteries is that we cannot recycle them by charging them again. Recycling becomes more important as batteries get larger and frequent battery replacement is not commercially viable.
One of the world’s first rechargeable batteries, the nickel-cadmium battery, also uses an alkaline electrolyte. In 1989, the nickel-metal hydride battery (NiMH) was invented, which has a longer life than the nickel-cadmium battery.
This type of battery is very sensitive to overcharging and overheating, so the charging power should be controlled below a maximum power. However, a well-designed controller can speed up the charging process, so we don’t have to wait for hours to charge.