New strategy for high energy density lithium batteries: fully electrochemically active all-solid-state lithium batteries
High energy density is an important development direction for energy storage devices in the future. Lithium-ion batteries, as a type of energy storage device with excellent performance, have made great achievements in the past few decades. However, the energy density of traditional lithium-ion battery cathode materials has already approached the theoretical value, and how to further improve the energy density has become a research hotspot that has attracted worldwide attention.
All-solid-state metal lithium batteries have attracted extensive attention from researchers as the next generation of high energy density mainstream technology solutions. In theory, the energy density of battery devices is determined by their theoretical energy density at the material level. However, at the electrode level, a large amount of inactive components (electrolytes, conductive additives and adhesives) need to be introduced to ensure the ion and electron transport capacity of the electrode material, so that the energy density at the electrode material level is usually less than the theoretical energy density of the material. The gap between the two is further widened in all-solid-state electrodes. Therefore, how to give full play to the theoretical energy density of the material at the electrode level is regarded as an important research direction.
Li Meiying, a doctoral student in the E01 group of the Clean Energy Laboratory of the Institute of Physics, Chinese Academy of Sciences/Beijing National Center for Condensed Matter Physics, worked with Professor Li Ju of the Massachusetts Institute of Technology under the guidance of Academician Chen Liquan and Distinguished Researcher Suo Liumin to propose for the first time a new idea of using all-electrochemically active electrodes to construct all-solid-state batteries. By using highly electron-ion mixed conductive active materials as the positive electrode to achieve a 100% all-active material all-solid-state electrode, and combining it with a metal lithium negative electrode, a high-energy-density all-active material all-solid-state battery was constructed. In this new type of all-solid metal lithium battery, the energy density at the material level can be 100% utilized at the electrode level.
The concept of fully electrochemically active all-solid-state batteries was first realized in a series of electrochemically active high ion-electron transition metal sulfide materials, and through the combination with high-capacity sulfur positive electrodes, an energy density of 770Wh/kg and 1900Wh/L was achieved at the electrode level (the energy density of commercial lithium cobalt oxide electrodes is 480 Wh/kg and 1600Wh/L). It is expected that with the discovery of more new fully active solid-state electrodes in the future, the energy density of all-solid-state batteries will be further improved, thus ultimately realizing high energy density and high safety all-solid-state lithium batteries.
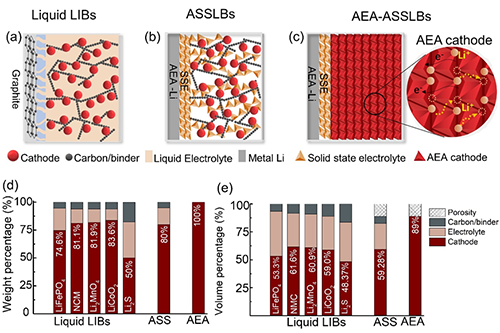
Figure 1. Concept of fully electrochemically active electrodes: a. Commercial lithium-ion liquid batteries (cathode: 74.6~83.6wt%; anode: graphite). b. Conventional ASSLBs (cathode: 80wt%; anode: lithium metal). c. Fully electrochemically active electrode (AEA, cathode: 100wt%; anode: metallic lithium). d and e: Summary table of weight and volume percentage of various components.
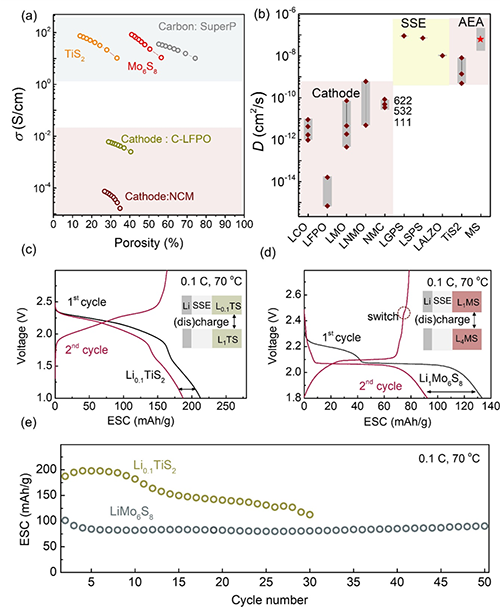
Figure 2. Proof of concept of AEA-ASSLBs: a. Comparison of electronic conductivity of AEA electrode with some traditional battery materials. b. Lithium ion diffusion coefficient of AEA material obtained by potentiostatic intermittent titration technique (PITT) and compared with existing traditional battery materials. c, d and e. Their cycling stability at 0.1C/70℃.
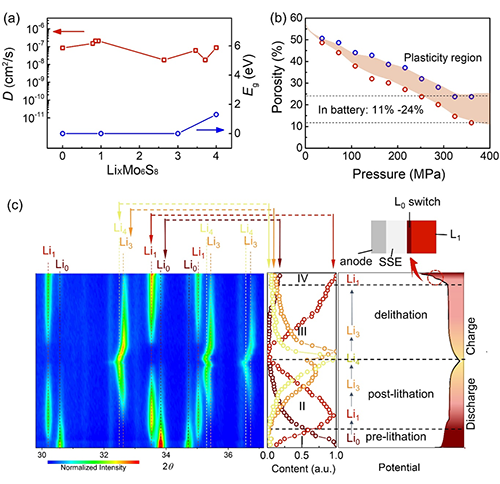
Figure 3. Electrochemical mechanism of AEA electrode based on LixMo6S8: a. Lithium ion diffusion coefficient and band gap of LixMo6S8 (x = 0, 1, 3, 4). b. Porosity of AEA Mo6S8 electrode as a function of applied pressure. Red circles represent pressure-applied value tests, and blue circles represent pressure-released value tests. c. Left figure shows in situ XRD analysis of LixMo6S8-based AEA electrode. Middle figure shows normalized peak intensity at different stages, accompanied by phase transition process of charge and discharge curves (right figure).
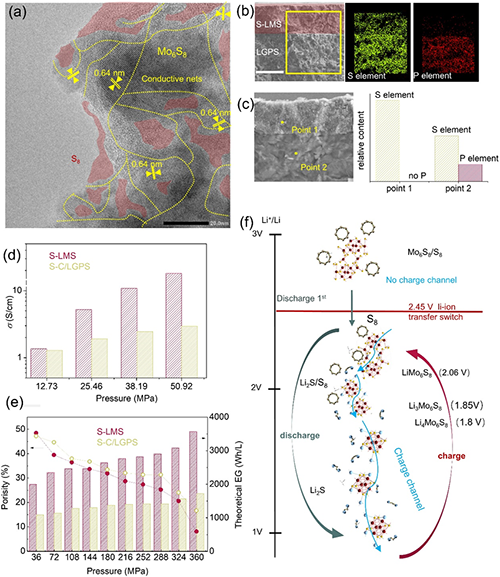
Figure 4. Structure and electrochemical mechanism of the composite S-LMS-AEA cathode (32.5%S8-67.5%Mo6S8). a. TEM image. b. Cross-sectional SEM of the AEA electrode. c. EDS analysis of P and S elements. The black scale bar is 20 is 50 μm, and the white scale bar is 50 μm. d and e: Comparison of electronic conductivity, theoretical volume energy density, and porosity of the S-LMS-AEA electrode with that of a typical S-C-LGPS cathode. f. Electrochemical redox mechanism of the S-LMS-AEA electrode.
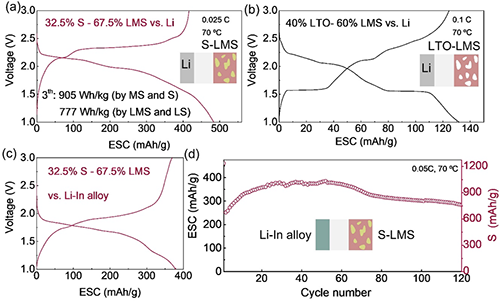
Figure 5. High energy density fully active solid-state battery strategy. a-c: Charge and discharge characteristics of all-solid-state batteries constructed with S-LMS (32.5% S8-67.5% Mo6S8) and Li, LTO-LMS (40% Li4Ti5O12-60% Mo6S8) and Li, and S-LMS and Li-In alloy. d. Cycling stability of AEA battery with S-LMS cathode.